i. TGA Disinfectant test
TGA
(Therapeutic Goods Administration) disinfectant test is required to support the claims of bactericidal efficacy
according to Australian standards. Therapeutic Goods (Standard for
Disinfectants and Sanitary Products) 2019 is designed to support the quality,
safety and efficacy of therapeutic goods that are disinfectants, sterilants,
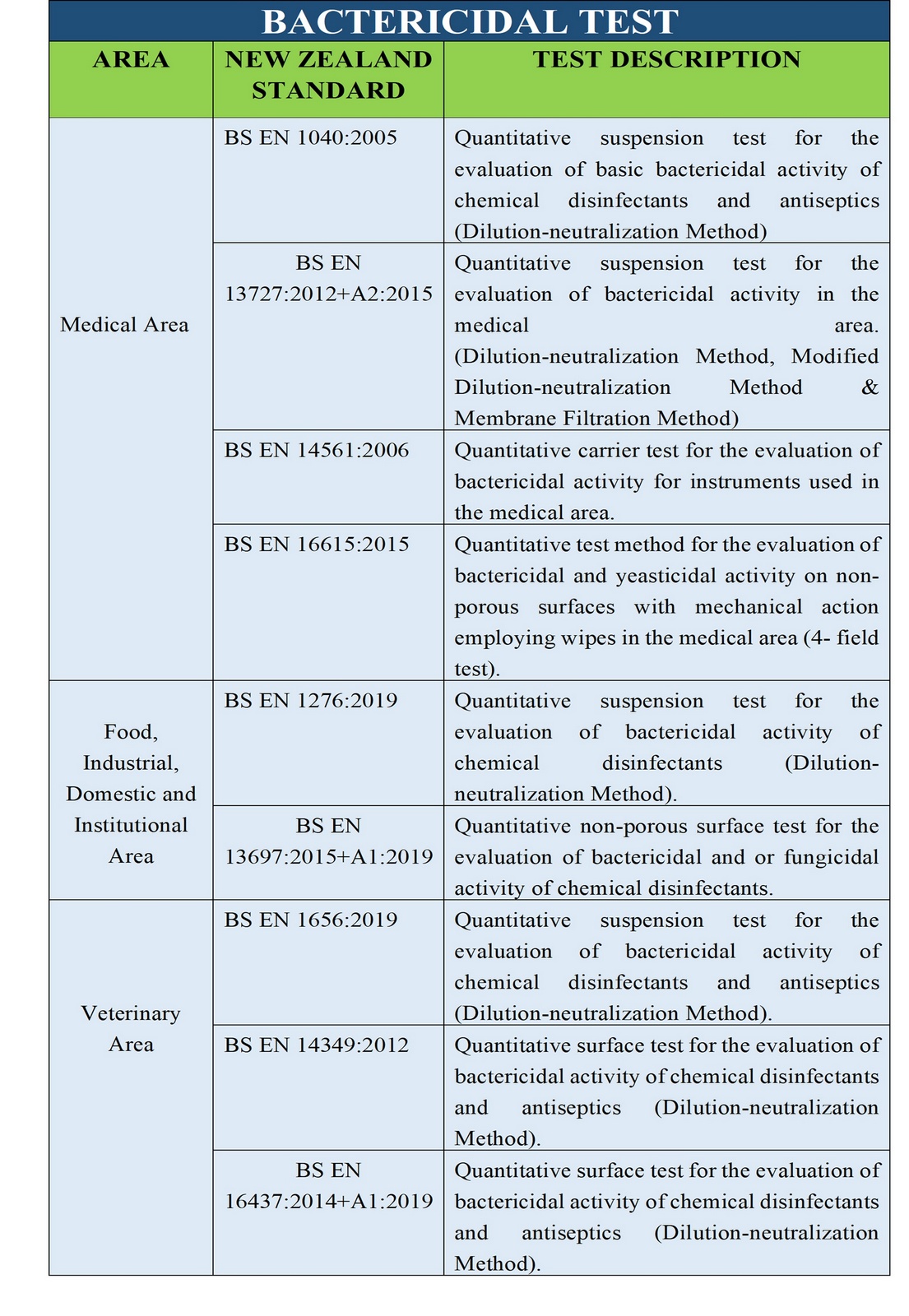
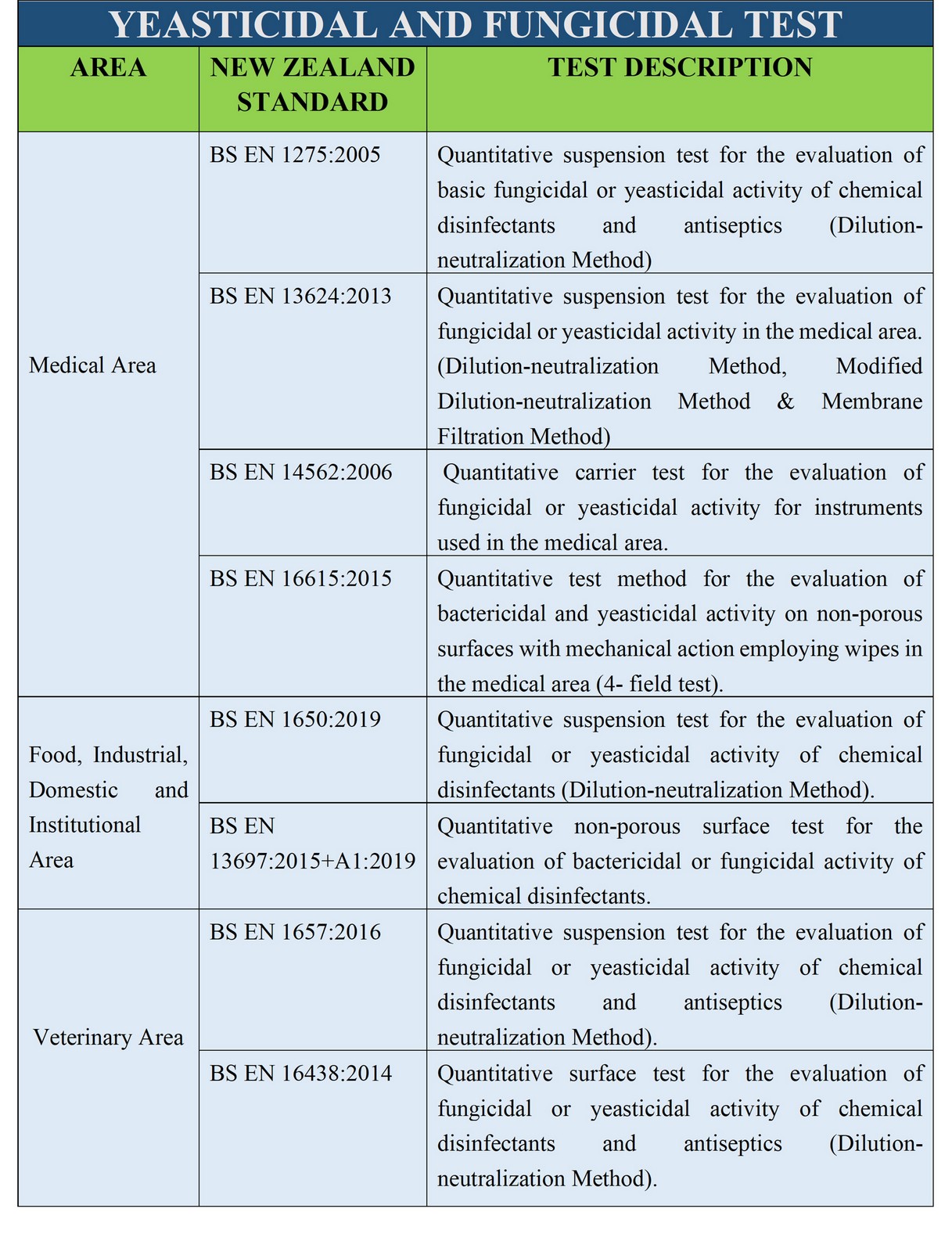
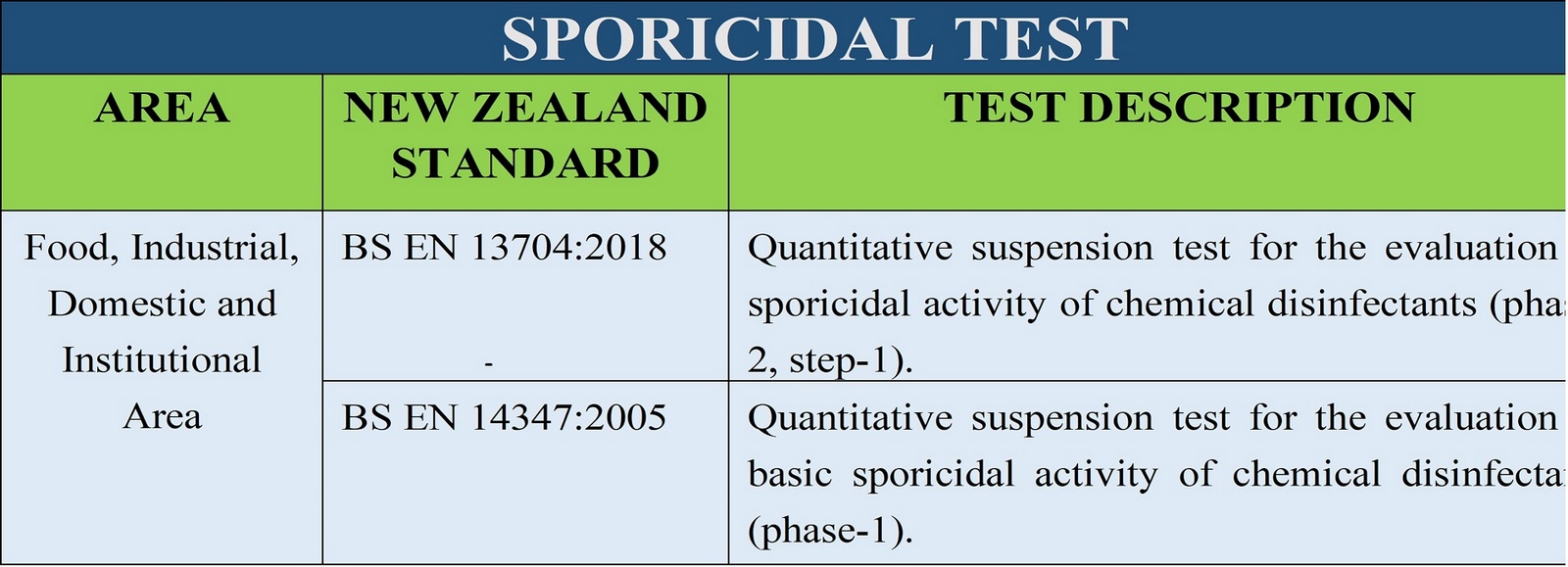
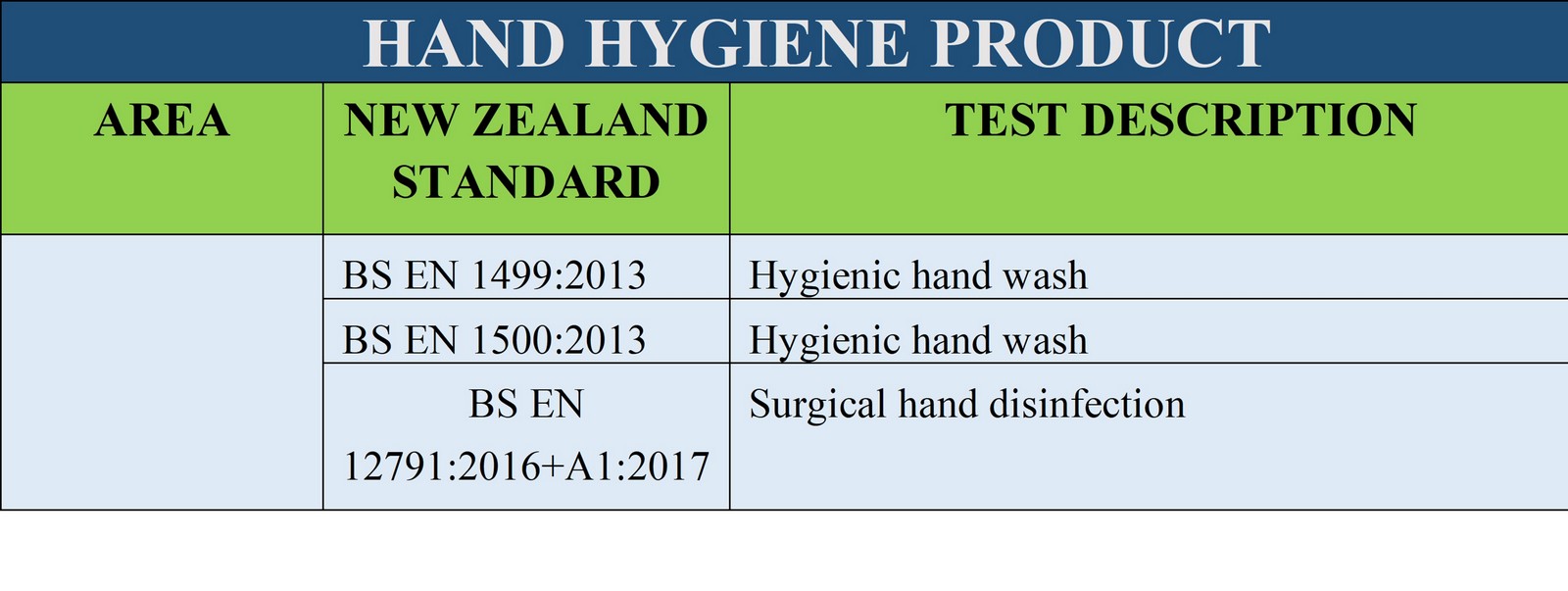
sanitary fluids and powders. TGA Disinfectant Test (1) refers to the suspension test
used in Australia to determine the bactericidal activity of disinfectants. TGA Disinfectant test should be
your first focus when expanding your disinfectant business. The manufacturing and marketing of disinfectants in
Australia are guided by Therapeutic Goods Order No. 104 (TGO 104).
The European equivalent of this
test includes
BS EN 13727:2012+A2:2015, BS EN
1656:2009 and BS EN 1276:2009.
i) For a
hospital-grade disinfectant
ii) For a household/commercial
grade disinfectant .